"like osteoblasts and osteocytes, MSCs are mechanoresponsive."<-with sufficient fluid flow we could conceivable stimulate the MSCs to become cells that make us taller.
"fluid flow would result in an increase in intracellular calcium concentration ([Ca2+]i) and the subsequent activation of downstream signaling proteins, such as ERK1/2 and calcineurin, which would in turn induce hMSC proliferation. is a vital and ubiquitous mediator in the processes by which extracellular signals are conveyed to the cell's interior and translated into a cellular response. Oscillations in [Ca2+]i regulate gene expression via numerous signaling cascades and have been shown to provide specificity among transcriptional activators. The serine/threonine protein phosphatase calcineurin, for instance, responds to increases in [Ca2+]i and calmodulin binding by dephosphorylating and activating nuclear factor of activated T cells (NFAT) transcription factors. Likewise, ERK1/2, members of the MAP kinase family, have been shown to be key regulators in the proliferation and differentiation of numerous cell types, including hMSCs and osteoblasts"
"During the static period, 1.4 ± 0.6% of cells exhibited a spontaneous increase in [Ca2+]i with an amplitude of 68.9 ± 9.1 nM. A significantly higher percentage of cells exhibited increases in [Ca2+]i when hMSCs were exposed to oscillatory fluid flow-inducing shear stresses of 5, 10, and 20 dyn/cm2 at 1 Hz (56 ± 2.4%, 87 ± 7.9%, and 89 ± 10.4%, respectively). Interestingly, the amplitudes of the observed [Ca2+]i increase in response to 5 and 10 dyn/cm2 (60.0 ± 10.2 and 96.8 ± 6.3 nM, respectively) were not statistically significantly different from spontaneous increases observed in static controls. In contrast, a significantly greater increase in [Ca2+]i was observed when cells were exposed to a shear stress of 20 dyn/cm2 (189 ± 12.4 nM)"<-it is possible that typical exercises do not generate sufficient fluid flow to induce the stimulus to make us and we need new exercises involving things like torsion to generate that stimulus.
"increased bone formation in response to mechanical loading is mediated by the response of bone cells to strain-induced fluid flow"<-since we see in this study other cells are stimulated too and these non bone cells could make you taller it's even conceivable that bone cells can make you taller.
Would increased interstitial fluid flow through in situ mechanical stimulation enhance bone remodeling?
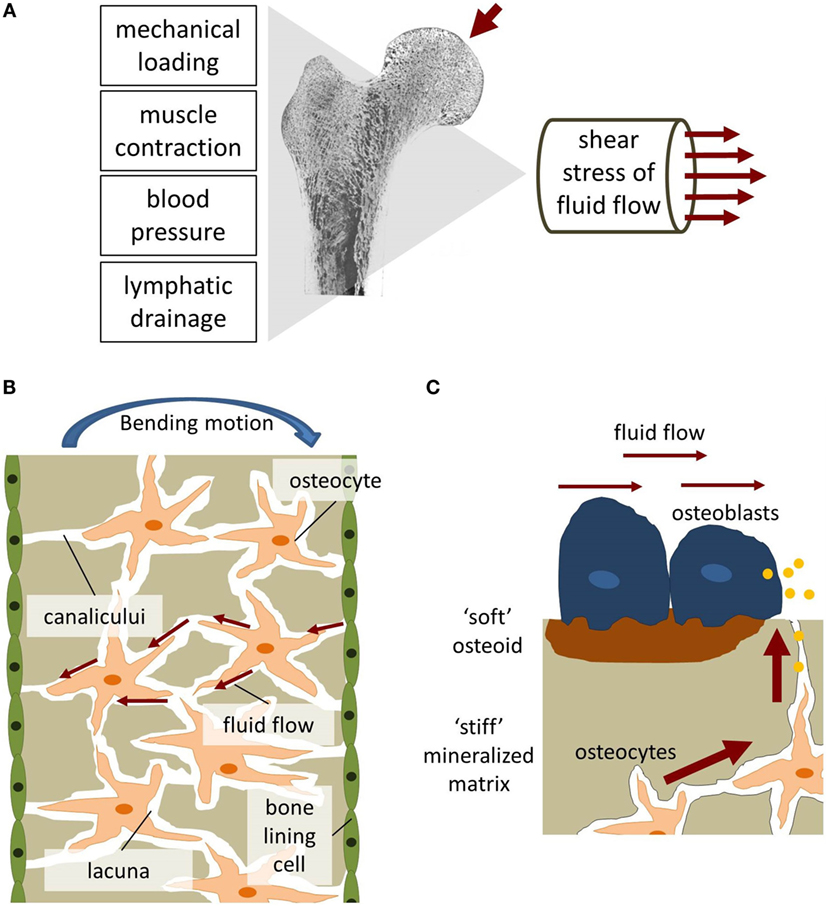
"Bone is a composite material made up of a collagen-hydroxyapatite matrix and a complex network of lacunae-canaliculi channels occupied by osteocyte and osteoblast processes, immersed in interstitial fluid. Changes in interstitial fluid flow velocity or pressure are the means by which an external load signal is communicated to the cell. Shear stress, induced by interstitial fluid flow, is a potent bone cell behavior regulator. One of the forms of altering interstitial fluid flow is through the mechanical deformation of skeletal tissue in response to applied loads[like via Lateral Synovial Joint Loading]. Other methods include increased intramedullary pressure, negative-pressure tissue regeneration, or external mechanical stimulation. The efficacy of each method theoretically will depend on the mechanical efficiency of transmitting an external load and converting it into changes in interstitial fluid flow. [A] small mechanical percussion device could be placed directly in contact with the bone, thus inducing local interstitial fluid flow variations."
"Within the confined geometry of the Haversian and lacunar–canalicular systems, interstitial fluid flow would impart shear stress upon cell membranes and processes. Fluid flow enhances cell proliferation and the expression of phenotypic markers of osteoblastic cells[it may enhance cellular proliferation of height increasing cells indirectly]. interstitial fluid flow induces the release of the paracrine factors[stem cells in the epiphysis may be able to benefit from this] necessary for the anabolic response of bone to mechanical loads. Fluid flow was also shown to increase osteocytic prostaglandins[prostaglandins like PGE2 has mixed effects on height growth] and nitric oxide[Nitric Oxide pathway is involved in things like Guanyl Cyclase and CNP which can increase height growth]"
Increasing osteoblastic differentiation may increase height in some bones dependent on the location of the periosteum such as the flat bone of the skull and the calcaneus.
"The efficiency of inducing interstitial fluid flow can be improved if mechanical stimuli could be applied directly to the bone surface or within the intramedullary canal through a small mechanical, low-power, percussion device with programmable frequency and amplitude. This method of delivering mechanical stimuli would be more efficient, given that the stimulation would be applied directly to the bone, eliminating the shock-absorbing properties of muscles and joints, as occurs when stimulation is applied externally."<-mechanical stimuli on bone is more effective when applied directly to the bone due to the shock-absorbing properties of muscles and joints. Hence, why individuals who lift extremely heavy weights like strong have not grown (much) taller. Lateral Synovial Joint Loading is only blocked by the skin.
If sufficient fluid flow in the cortical bone requires direct mechanical loading to the bone it is likely that sufficient fluid build up(hydrostatic pressure) in the epiphysis requires direct mechanical loading as well.
Here's one study that shows how an increase in intramedullary pressure increase bone length(the mice were 8 weeks old):
Femoral vein ligation increases bone mass in the hindlimb suspended rat.
"Interstitial fluid flow (IFF) [is] due to intraosseous pressure changes. [We investigated] the role of IFF in bone in the absence of mechanical strain using an in vivo model, the hindlimb suspended rat. Ligation of one femoral vein{veins take blood towards the heart while artieries take blood away from the heart so removing a vein would result in more blood in the limbs} was performed as a means to alter the IFF within the ipsilateral femur; the contralateral limb was sham-operated as control. Animals were suspended for a period of 19 days. Intramedullary pressure in the venous-ligated femurs increased relative to the sham-operated control femurs (27.8 mmHg vs. 16.4 mmHg), suggesting venous ligation increased IFF proportional to the pressure drop across the bone. Bone mineral content (BMC), when normalized to body weight, increased significantly in the venous-ligated femurs relative to control limbs (115.9 +/- 15.6% vs. 103.8 +/- 13.2%); similarly, gains in length (106.2 +/- 2.4% vs. 104.5 +/- 2.1%) and distal width (110.8 +/- 10.3% vs. 106.2 +/- 8.2%, p < 0.05) for the femurs with venous ligation were significantly greater relative to sham control. Furthermore, trabecular density was significantly higher in the femurs with venous ligation (351 +/- 12 g/cm3 vs. 329 +/- 11 g/cm3, p < 0.05). Daily administration of the cyclooxygenase inhibitor, indomethacin, via drinking water, suppressed the length increases observed for the venous ligated femur, suggesting a role for prostaglandins in IFF-mediated remodeling[COX2 was upregulated by LSJL as PTGS2]."
"Bone contains a porous network of canaliculi that has been shown to facilitate substantial and rapid transcortical interstitial fluid flow (IFF). This fluid flow originates from leaky venous sinusoids in the intramedullary cavity and is driven radially outward through cortical bone by a transmural pressure gradient between the endosteal vasculature and the lymphatic drainage at the periosteal surface. Flow is steady in the absence of mechanical strain, but bending or compressive loads create pressure gradients that drive fluid from areas of compression to areas of tension[laterally loading the epiphysis generates a pressure gradient down the entire bone including through the growth plate]."
Ligation refers to tying off veins.
"A wire approximately twice the length of the tail was then folded in half and positioned on the tail such that wire ran along each side and the apex was at the dorsal tip of the tail. The wire was then secured in this position with athletic tape so that it was sandwiched between two layers. This wire was attached to a swivel hook from which the animal was suspended. With the hindlimbs fully outstretched, the feet were approximately 1 cm off the ground. Suspension lasted for 19 days."<-the mice were suspended. Inversion essentially.
"Relative length and relative distal width of the venous-ligated limb was significantly greater in the HS rat compared with the sham limb"<-the hindlimb suspended mice grew taller. Although, only the mice who had their veins tied off.
"Femoral vein occlusion has previously been shown to result in an increase in intramedullary pressure"<-So the mice were suspended to eliminate mechanical loading. The height gain was due to the increase in intramedullary pressure and not another effect of mechanical loading. Although an increase in intramedullary pressure would logically coincide with an increase in hydrostatic pressure in the epiphysis.
According to the study Growth of the Laboratory Mouse, mice start to slow down growth at day 42(6 weeks) but continue growing through day 98(14 weeks) so the height growth could be a modulation of hydrostatic pressure on the tail growth plates rather than new stem cell differentiation. And it's unclear whether this is an increase in growth rate or absolute growth. The rats in this study were female and 8 weeks old.
Maybe it's not intramedullary pressure that increased the bone length after all and it's the increase in prostaglandins in the interstitial fluid that cause an increase in length(it could still just be an increase in growth rate mind you). But, inhibiting COX2(which is involved in PGE2 expression) only suppresed the length increase, it didn't totally eliminate it thus a change in intramedullary pressure has an effect independent of increased transport of PGE2 through the interstitial fluid. Such as perhaps an induction of mesenchymal stem cells into chondrocytes.
Here's a study that explains the path of Bone fluid flow so we can analyze how much fluid flow goes to the epiphysis:
The pathway of bone fluid flow as defined by in vivo intramedullary pressure and streaming potential measurements.
"The pathway for intracortical fluid flow response to a step-load was identified in vivo using intramedullary pressure (ImP) and streaming potential (SP) measurements. An avian model was used for monitoring, simultaneously, ImP and SP under axial loading which generated peak strains of approximately 600 microstrain (microepsilon). ImP response to step-load decayed more quickly[thus you may have to load for a long time to keep Intramedullary Pressure high to induce chondrogenesis] than SP relaxation, in which multiple time constants were observed during the relaxations. While the initial relaxation of SP showed a decay on the order of 200 ms, ImP decayed on the order of approximately 100 ms[this is not very long at all so it's possible that LSJL provides must of it's growth stimulating benefits when it's being applied and there are low residual effects after]. After the initial decay (approximately 200 ms after loading), ImP quickly relaxed to base line, while SP continued to dominate relaxation. The decay of ImP is indicative of resistive fluid flow occurring primarily in the vasculature and other intraosseous channels such as lacunar-canalicular pores, and that SP represents the fluid flow in the smaller porosities, i.e., lacunar-canalicular system or even microspores[Since the epiphysis is mostly trabeculae it is not dense so it is likely to belong to the group that decays very quickly]. SP and ImP decays are determined by a hierarchical interdependent system of multiple porosities."
"mechanically induced deformation of bone acts as a motive force for fluid displacement, generating fluid pressure gradients that drive interstitial fluid into the LCS and bony macrostructure. The forces generated act directly on bone cells, and this load-induced fluid flow is critical for mechanotransduction as well as enhancing convective solute transport within the macro- and microporosities."
"fluid shear stress can induce deformation of the bone cell membrane and alteration of membrane proteins, opening mechano-activated ion channels to allow the influx of cations, such as Ca2+, Na+, and K+, into the cell. Stretch-activated ion channels include the DEC/ENAC family of cation channels (named after Caenorhabditis elegans degenerins and mammalian Na+ channels), L-type (osteoblasts) and T-type (osteocytes) voltage-sensitive calcium channels, and annexin V voltage-gated calcium channels. Specifically, increases in osteoblast, osteocyte, and MSC membrane tension induce the opening of PIEZO1 channels. Mechanically activated nonselective Ca2+-permeable cation channels of the PIEZO family (PIEZO1 and PIEZO2) are recognized as the most important mediators of mechanotransduction and are crucial for bone formation. Mechanical stimulation enhances calcium flux into the cell, and the resulting calcium spikes mediate osteogenesis."<-I believe that it can stimulate other kinds of issue formation as well.
" Human bone marrow-derived stem cells respond to active mechanical stimulation, where 2%-8% uniaxial strain through tensile stretching resulted in osteogenic differentiation or subsequent bending resulted in both osteogenic and chondrogenic differentiation"<-Yes exactly chondrogenic differentiation! This is what can impact height.
"in normogravity, deformation of the pores during loading induces motion in the fluid-like marrow, resulting in the generation of pressure and velocity gradients"
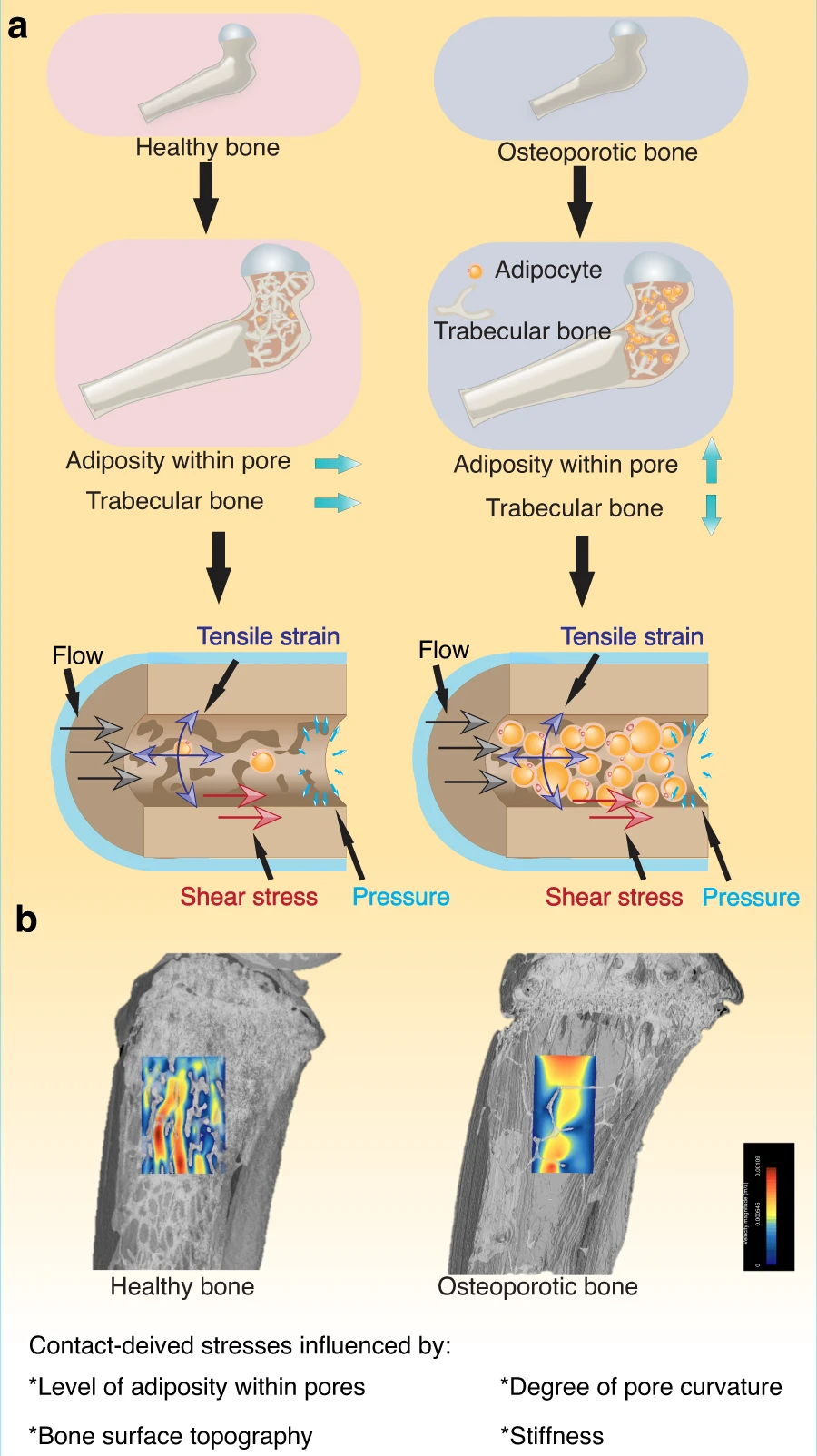
"a reduced bone volume resulted in an overall increase in bone deformation, leading to increased stimulation via microstrain to cells. Furthermore, an increased adipocyte content in the marrow resulted in lowering the microstrain levels to cells within the bone marrow, reportedly due to a shielding effect caused by the more compliant behavior of adipocytes."
In Vitro Bone Cell Models: Impact of Fluid Shear Stress on Bone Formation
" Shear stress generated by interstitial fluid flow in the lacunar-canalicular network influences maintenance and healing of bone tissue. Fluid flow is primarily caused by compressive loading of bone as a result of physical activity{I believe that torsional loading is the best way to generate fluid flow}. Changes in loading, e.g., due to extended periods of bed rest or microgravity in space are associated with altered bone remodeling and formation in vivo. In vitro, it has been reported that bone cells respond to fluid shear stress by releasing osteogenic signaling factors, such as nitric oxide, and prostaglandins. This work focusses on the application of in vitro models to study the effects of fluid flow on bone cell signaling, collagen deposition, and matrix mineralization. Particular attention is given to in vitro set-ups, which allow long-term cell culture and the application of low fluid shear stress. In addition, this review explores what mechanisms influence the orientation of collagen fibers, which determine the anisotropic properties of bone. A better understanding of these mechanisms could facilitate the design of improved tissue-engineered bone implants or more effective bone disease models."
"Bone has the power to regenerate and repair constantly throughout the entire life."
"MSCs differentiate into osteoblasts under the appropriate stimuli, but they can also turn into cartilage, muscle, tendon, and fat cells"
"Osteocytes are terminally differentiated osteoblasts which became trapped within newly deposited bone matrix "
"Osteocytes, which are the most abundant cell type in bone (90–95% of total bone cells), are thought to respond to mechanical loading by releasing signal factors."
"mineralization is likely to be controlled by the small Ca2+-binding protein osteocalcin. Mechanotransduction is facilitated by glycoproteins, such as osteopontin and osteonectin, which can attach to integrins on cell surfaces. Osteopontin also enables the attachment of osteoclasts to bone surfaces. The small amount of lipids is crucial for cell signaling and ion flow"
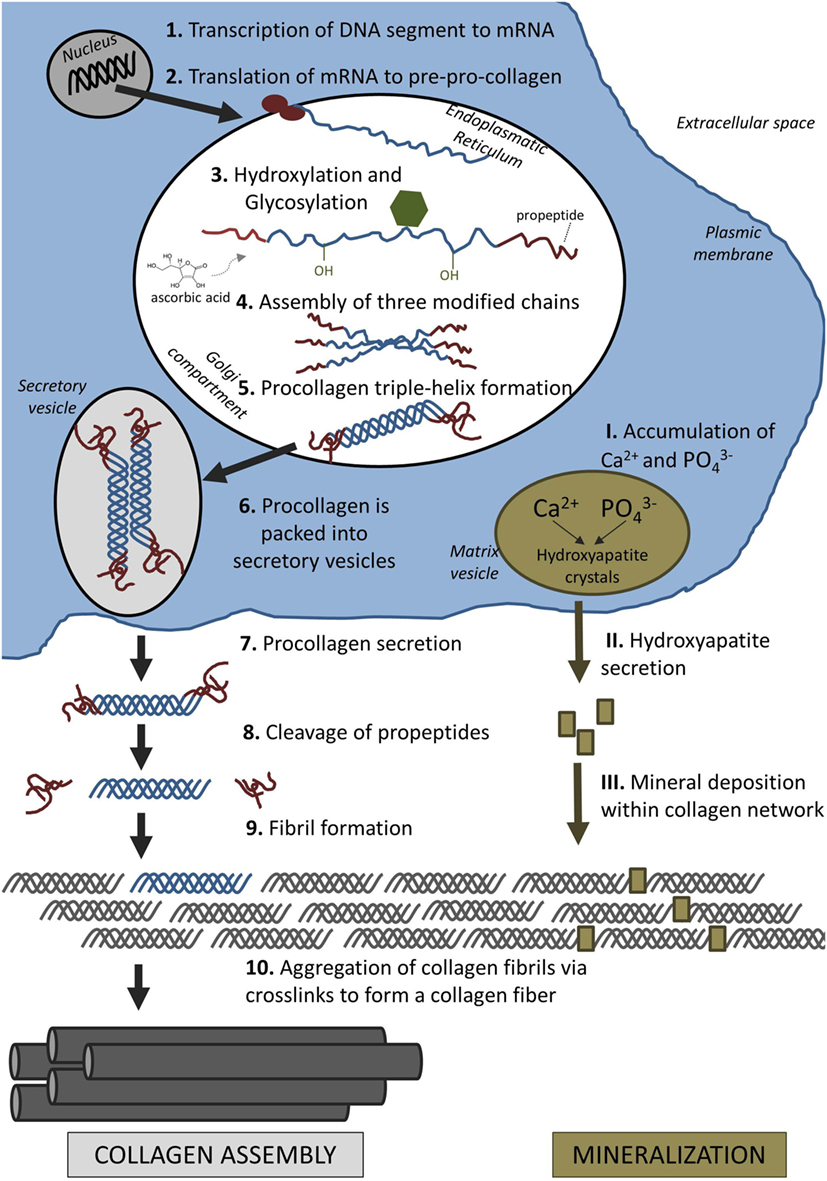
"Collagen formation is initiated in the nucleus of collagen-producing cells, such as osteoblasts and also fibroblasts. In the nucleus, a particular segment of deoxyribonucleic acid (DNA) is transcribed into messenger ribonucleic acid (mRNA). After the mRNA has moved out of the nucleus into the cytoplasm, it is translated into polypeptide chains, known as pre-pro-collagen. Each chain is about 300 nm in length and 1.5 nm in diameter. They are characterized by a strict pattern consisting of multiple triplet sequences of Gly–Y–Z. Glycine residues (Gly) have to be present in every third position to allow proper folding of these chains later on. Although Y and Z can be any amino acid, they are commonly proline and hydroxyproline"
"Proline and lysine residues are then hydroxylated in the endoplasmatic reticulum (ER) which will aid cross-linking of peptide chains later. This enzymatic step requires ascorbic acid (vitamin C) as a cofactor. A lack of ascorbic acid would either result in the formation of looser collagen triple helices or prevent collagen synthesis altogether, resulting in diseases such as scurvy"
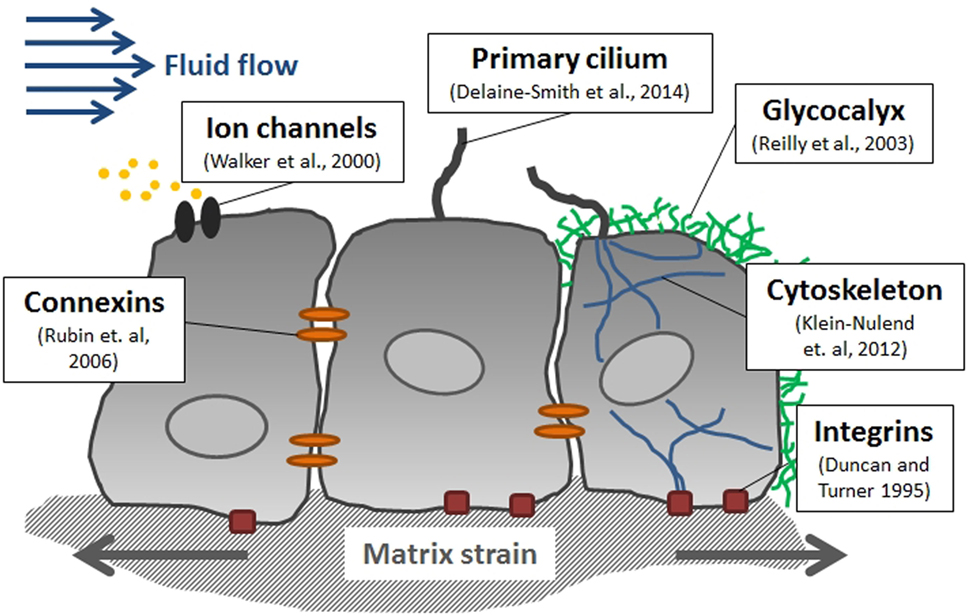
"The matrix-integrin-cytoskeleton pathway is thought to play an important role in bone mechanotransduction, since integrins directly connect bone cells with their ECM. Integrins are membrane-bound glycoproteins which allow rapid transmission of physical stimuli from the ECM via the cytoskeleton to the nucleus, where they could initiate changes in gene expression"
" The glycocalyx, which is a cellular coating rich in hyaluronic acid, might also contribute to bone cell mechanotransduction via force transmission to the cytoskeleton and integrins"
"Interstitial fluid (ISF) is a main component of body mass (up to 20%) and is distributed throughout the ECM. It provides cells with nutrients and waste removal and can also be found in cortical and cancellous bone where it fills the porosities within the tissue. The three levels of porosities in bone are: (1) the vascular porosity within the Volkmann canal and the Haversian canals (20 μm radius), (2) the lacunar-canalicular system (LCS), which are the channel structures within the mineralized bone tissue surrounding osteocytes and their processes (0.1 μm radius), and (3) tiny spaces between crystallites of the mineral hydroxapatite and collagen fibers (0.01 μm radius)"
"Mechanical loading results in bending of bones and matrix deformation. Compressive stress is generated on one side of the bone and tensile stress on the other. The resulting pressure gradient in the ISF is thought to drive the fluid from regions of compression to tension. ISF has to squeeze through the narrow channels, canaliculi, which connect osteocytes residing in small spaces, lacunae, within the mineralized bone matrix. Due to the small dimensions of the channels, high wall shear stress comparable to vascular wall shear stress is generated"
"Osteocytes subjected to FSS have been shown to release several physiological relevant messengers in vitro, including Ca2+, ATP, NO, and PGE2"
Osteocyte Control of Osteoclastogenesis
"osteocytes can directly control the differentiation and activity of either osteoclasts or osteoblasts."
"Osteocyte apoptosis also increases in response to changes in biomechanical loading of the skeleton. Interestingly, both over-loading and unloading of the skeleton stimulate osteocyte apoptosis and both conditions result in increased bone resorption"
"apoptotic bodies produced by dying osteocyte-like cells in vitro are able to promote osteoclast formation in vitro and in vivo"
"Bone is a composite material made up of a collagen-hydroxyapatite matrix and a complex network of lacunae-canaliculi channels occupied by osteocyte and osteoblast processes, immersed in interstitial fluid. Changes in interstitial fluid flow velocity or pressure are the means by which an external load signal is communicated to the cell. Shear stress, induced by interstitial fluid flow, is a potent bone cell behavior regulator. One of the forms of altering interstitial fluid flow is through the mechanical deformation of skeletal tissue in response to applied loads[like via Lateral Synovial Joint Loading]. Other methods include increased intramedullary pressure, negative-pressure tissue regeneration, or external mechanical stimulation. The efficacy of each method theoretically will depend on the mechanical efficiency of transmitting an external load and converting it into changes in interstitial fluid flow. [A] small mechanical percussion device could be placed directly in contact with the bone, thus inducing local interstitial fluid flow variations."
"Within the confined geometry of the Haversian and lacunar–canalicular systems, interstitial fluid flow would impart shear stress upon cell membranes and processes. Fluid flow enhances cell proliferation and the expression of phenotypic markers of osteoblastic cells[it may enhance cellular proliferation of height increasing cells indirectly]. interstitial fluid flow induces the release of the paracrine factors[stem cells in the epiphysis may be able to benefit from this] necessary for the anabolic response of bone to mechanical loads. Fluid flow was also shown to increase osteocytic prostaglandins[prostaglandins like PGE2 has mixed effects on height growth] and nitric oxide[Nitric Oxide pathway is involved in things like Guanyl Cyclase and CNP which can increase height growth]"
Increasing osteoblastic differentiation may increase height in some bones dependent on the location of the periosteum such as the flat bone of the skull and the calcaneus.
"The efficiency of inducing interstitial fluid flow can be improved if mechanical stimuli could be applied directly to the bone surface or within the intramedullary canal through a small mechanical, low-power, percussion device with programmable frequency and amplitude. This method of delivering mechanical stimuli would be more efficient, given that the stimulation would be applied directly to the bone, eliminating the shock-absorbing properties of muscles and joints, as occurs when stimulation is applied externally."<-mechanical stimuli on bone is more effective when applied directly to the bone due to the shock-absorbing properties of muscles and joints. Hence, why individuals who lift extremely heavy weights like strong have not grown (much) taller. Lateral Synovial Joint Loading is only blocked by the skin.
If sufficient fluid flow in the cortical bone requires direct mechanical loading to the bone it is likely that sufficient fluid build up(hydrostatic pressure) in the epiphysis requires direct mechanical loading as well.
Here's one study that shows how an increase in intramedullary pressure increase bone length(the mice were 8 weeks old):
Femoral vein ligation increases bone mass in the hindlimb suspended rat.
"Interstitial fluid flow (IFF) [is] due to intraosseous pressure changes. [We investigated] the role of IFF in bone in the absence of mechanical strain using an in vivo model, the hindlimb suspended rat. Ligation of one femoral vein{veins take blood towards the heart while artieries take blood away from the heart so removing a vein would result in more blood in the limbs} was performed as a means to alter the IFF within the ipsilateral femur; the contralateral limb was sham-operated as control. Animals were suspended for a period of 19 days. Intramedullary pressure in the venous-ligated femurs increased relative to the sham-operated control femurs (27.8 mmHg vs. 16.4 mmHg), suggesting venous ligation increased IFF proportional to the pressure drop across the bone. Bone mineral content (BMC), when normalized to body weight, increased significantly in the venous-ligated femurs relative to control limbs (115.9 +/- 15.6% vs. 103.8 +/- 13.2%); similarly, gains in length (106.2 +/- 2.4% vs. 104.5 +/- 2.1%) and distal width (110.8 +/- 10.3% vs. 106.2 +/- 8.2%, p < 0.05) for the femurs with venous ligation were significantly greater relative to sham control. Furthermore, trabecular density was significantly higher in the femurs with venous ligation (351 +/- 12 g/cm3 vs. 329 +/- 11 g/cm3, p < 0.05). Daily administration of the cyclooxygenase inhibitor, indomethacin, via drinking water, suppressed the length increases observed for the venous ligated femur, suggesting a role for prostaglandins in IFF-mediated remodeling[COX2 was upregulated by LSJL as PTGS2]."
"Bone contains a porous network of canaliculi that has been shown to facilitate substantial and rapid transcortical interstitial fluid flow (IFF). This fluid flow originates from leaky venous sinusoids in the intramedullary cavity and is driven radially outward through cortical bone by a transmural pressure gradient between the endosteal vasculature and the lymphatic drainage at the periosteal surface. Flow is steady in the absence of mechanical strain, but bending or compressive loads create pressure gradients that drive fluid from areas of compression to areas of tension[laterally loading the epiphysis generates a pressure gradient down the entire bone including through the growth plate]."
Ligation refers to tying off veins.
"A wire approximately twice the length of the tail was then folded in half and positioned on the tail such that wire ran along each side and the apex was at the dorsal tip of the tail. The wire was then secured in this position with athletic tape so that it was sandwiched between two layers. This wire was attached to a swivel hook from which the animal was suspended. With the hindlimbs fully outstretched, the feet were approximately 1 cm off the ground. Suspension lasted for 19 days."<-the mice were suspended. Inversion essentially.
"Relative length and relative distal width of the venous-ligated limb was significantly greater in the HS rat compared with the sham limb"<-the hindlimb suspended mice grew taller. Although, only the mice who had their veins tied off.
"Femoral vein occlusion has previously been shown to result in an increase in intramedullary pressure"<-So the mice were suspended to eliminate mechanical loading. The height gain was due to the increase in intramedullary pressure and not another effect of mechanical loading. Although an increase in intramedullary pressure would logically coincide with an increase in hydrostatic pressure in the epiphysis.
According to the study Growth of the Laboratory Mouse, mice start to slow down growth at day 42(6 weeks) but continue growing through day 98(14 weeks) so the height growth could be a modulation of hydrostatic pressure on the tail growth plates rather than new stem cell differentiation. And it's unclear whether this is an increase in growth rate or absolute growth. The rats in this study were female and 8 weeks old.
Maybe it's not intramedullary pressure that increased the bone length after all and it's the increase in prostaglandins in the interstitial fluid that cause an increase in length(it could still just be an increase in growth rate mind you). But, inhibiting COX2(which is involved in PGE2 expression) only suppresed the length increase, it didn't totally eliminate it thus a change in intramedullary pressure has an effect independent of increased transport of PGE2 through the interstitial fluid. Such as perhaps an induction of mesenchymal stem cells into chondrocytes.
Here's a study that explains the path of Bone fluid flow so we can analyze how much fluid flow goes to the epiphysis:
The pathway of bone fluid flow as defined by in vivo intramedullary pressure and streaming potential measurements.
"The pathway for intracortical fluid flow response to a step-load was identified in vivo using intramedullary pressure (ImP) and streaming potential (SP) measurements. An avian model was used for monitoring, simultaneously, ImP and SP under axial loading which generated peak strains of approximately 600 microstrain (microepsilon). ImP response to step-load decayed more quickly[thus you may have to load for a long time to keep Intramedullary Pressure high to induce chondrogenesis] than SP relaxation, in which multiple time constants were observed during the relaxations. While the initial relaxation of SP showed a decay on the order of 200 ms, ImP decayed on the order of approximately 100 ms[this is not very long at all so it's possible that LSJL provides must of it's growth stimulating benefits when it's being applied and there are low residual effects after]. After the initial decay (approximately 200 ms after loading), ImP quickly relaxed to base line, while SP continued to dominate relaxation. The decay of ImP is indicative of resistive fluid flow occurring primarily in the vasculature and other intraosseous channels such as lacunar-canalicular pores, and that SP represents the fluid flow in the smaller porosities, i.e., lacunar-canalicular system or even microspores[Since the epiphysis is mostly trabeculae it is not dense so it is likely to belong to the group that decays very quickly]. SP and ImP decays are determined by a hierarchical interdependent system of multiple porosities."
"Fuid can pass within the lacunar-canalicular porosity[this is defined as the space between the lacunae and the canaliculae, these two terms describe any open space in bone and the epiphysis has tons of them]"<-Thus fluid can pass through the epiphysis.
"Penetration of fluid in bone can be enhanced by dynamic mechanical loading"
"An increase in intramedullary pressure can influence bone’s fluid pathways through several coupling mechanisms. First, as the pressure in the medullary cavity increases towards the arterial blood pressure, fluid flow into the marrow cavity will be greatly inhibited and perhaps even stopped. However, the increase in fluid velocity out of the medullary canal will relieve much of the pressure buildup associated with dynamic loading[it's unknown however the pressure build that occurs in the bone epiphysis however fluid velocity out of the medullary canal should impact the epiphysis]. The outward flow does not provide complete compensation for this relief mechanism, and thus high marrow cavity pressures arise immediately after step loading.
Second, considering that deformation of bone results in compression of the porous space within the cortical matrix, induced pore pressure is predicted at least one order of magnitude higher than load induced marrow cavity pressure. This results in the kinematic loading induced fluid pressure buildup in cortical bone being much greater than that achieved within the marrow cavity, though net fluid motion may be far less. This implies the existence of a fluid-related coupling mechanism. When the ability of fluid to leave the intracortical pores is restricted because of the deformation of the pore space under loading, the marrow cavity may still provide a pathway for relief of the intracortical pressure because of the pressure difference between porous matrix and marrow cavity. Thus, while induced marrow cavity pressure can potentially reduce the fluid flow, the fluid relaxation pathways are still active, especially during unloading, in both venous and intracortical pores."
The epiphysis should be deformed as well but the pressure of the epiphysis should be much less than that of the cortical bone so fluid should flow into the epiphysis thus increasing hydrostatic pressure there.
Here's a study that involves dynamic pressure stimulating growth, it involves fractured bones but some of the principles may apply.
The influence of intermittent external dynamic pressure and tension forces on the healing of an epiphyseal fracture.
"The lacunae-canaliculi system (LCS) within bone tissue and its anatomical parameters vary according to bone type, location, age and health. The LCS is composed of larger lacunae (~10 µm) and smaller canaliculi (0.1–0.5 µm) inhabited by osteocytes, and this porous system facilitates the exchange of substances, with liquid flow providing nutrients, eliminating metabolic waste and generating fluid shear force to stimulate osteocyte viability and function."
Here's a study that involves dynamic pressure stimulating growth, it involves fractured bones but some of the principles may apply.
The influence of intermittent external dynamic pressure and tension forces on the healing of an epiphyseal fracture.
"the regenerative capacity of chondrocytes located in the growth plate of long bones revealed a potential for reparation[perhaps what they actually observed was an ability for MSCs to differentiate into chondrocytes?]. Newly formed bridging arteries crossing from the metaphysis to the epiphysis through the growth plate are thought to be responsible for the cell proliferation observed after Salter-Harris I and II lesions. We aimed to examine the influence of mechanical microstimulations on the growth or inhibition of the proliferation of the chondrocytes in the tibial growth plate. Cell proliferation in the growth plate was not stimulated in the 1st week after distraction. The histological studies revealed an initial increase in proliferation of chondrocytes, especially between the 2nd and the 4th week."
"Chondrocytes exposed to relatively minimal intermittent tension and pressure react with an increased calcium and phosphate uptake"<-This means that if tension does not increase height but pressure does that increased calcium and phosphate uptake in chondrocytes is not the cause of the height increase.
"Fracture healing was completed histologically after 4 weeks. Immediately after induction of the fracture and stabilization of the fragment, no increase of the thickness of the specific cellular zones was seen, and no proliferation activity could be observed."<-so increase in pressure(can be induced by LSJL) had no height increasing impact until after the first week of fracture healing. At that point the bone may not have been repaired enough to generate enough pressure to stimulate growth. After the first week proliferative and hypertrophic zone were stimulated.
"Premature closure was frequently observed after epiphyseal distraction with a consequent loss of the bone length obtained."
"Arteries crossing from the metaphysis to the epiphysis through the growth plate are thought to be destroyed by the Salter-Harris lesions. These bridging capillaries must regrow after traumatic damage. They are important and responsible for cell proliferation, especially in the proliferative zone."
"Stimulation of the proliferative activity observed in our experiment i.e., induction of chondrogenesis in the proliferative and hypertrophic zones seems to be enhanced by the mechanical influence of the applied intermittent dynamic tension and pressure forces."
"Intermittent and alternating tension and pressure microstimulation of injuries of the growth plate and fractures of the growth region may be advantageous for the restoration of the injured growth plate."<-and in growth plates that are not injured and in people that do not have growth plates to form new ones.
Mechanically induced osteogenic differentiation--the role of RhoA, ROCKII and cytoskeletal dynamics.
"We examined whether oscillatory fluid flow, an exogenous mechanical signal within bone, regulates osteogenic, adipogenic or chondrogenic differentiation of C3H10T1/2 murine mesenchymal stem cells by measuring Runx2, PPARgamma and SOX9 gene expression, respectively. The small GTPase RhoA and isometric tension within the actin cytoskeleton are essential in flow-induced differentiation. oscillatory fluid flow induces the upregulation of Runx2, Sox9 and PPARgamma[fluid flow is definitely induced by LSJL and fluid flow upregulates Sox9]. the small GTPase RhoA and its effector protein ROCKII regulate fluid-flow-induced osteogenic differentiation. Activated RhoA and fluid flow have an additive effect on Runx2 expression. RhoA activation and actin tension are negative regulators of both adipogenic and chondrogenic differentiation. An intact, dynamic actin cytoskeleton under tension is necessary for flow-induced gene expression[this includes Sox9]."
There was no sign of RhoA activation in the LSJL gene expression study. Nor Roc II.
"C3H10T1/2 mesenchymal progenitor cells [were exposed] to 1 hour of oscillatory fluid flow"
"Cytoskeletal mechanics and isometric tension within the actin cytoskeleton alters oscillatory fluid-flow-induced differentiation by the activation of RhoA, inhibition of ROCKII protein, inhibition of myosin II ATP hydrolysis, disruption of actin polymerization, and actin stabilization."
"Although slow, fluid flow nevertheless plays an important role in nutrient transport, soft tissue maintenance and remodeling, as well as the establishment and maintenance of the microenvironment, where limitations in the supply of vital nutrients lead either to tissue adaptation or necrosis."<-soft tissue maintenance and remodeling could increase height via creation of tissue that is capable of interstitial growth.
Mechanically induced osteogenic differentiation--the role of RhoA, ROCKII and cytoskeletal dynamics.
"We examined whether oscillatory fluid flow, an exogenous mechanical signal within bone, regulates osteogenic, adipogenic or chondrogenic differentiation of C3H10T1/2 murine mesenchymal stem cells by measuring Runx2, PPARgamma and SOX9 gene expression, respectively. The small GTPase RhoA and isometric tension within the actin cytoskeleton are essential in flow-induced differentiation. oscillatory fluid flow induces the upregulation of Runx2, Sox9 and PPARgamma[fluid flow is definitely induced by LSJL and fluid flow upregulates Sox9]. the small GTPase RhoA and its effector protein ROCKII regulate fluid-flow-induced osteogenic differentiation. Activated RhoA and fluid flow have an additive effect on Runx2 expression. RhoA activation and actin tension are negative regulators of both adipogenic and chondrogenic differentiation. An intact, dynamic actin cytoskeleton under tension is necessary for flow-induced gene expression[this includes Sox9]."
There was no sign of RhoA activation in the LSJL gene expression study. Nor Roc II.
"C3H10T1/2 mesenchymal progenitor cells [were exposed] to 1 hour of oscillatory fluid flow"
"Cytoskeletal mechanics and isometric tension within the actin cytoskeleton alters oscillatory fluid-flow-induced differentiation by the activation of RhoA, inhibition of ROCKII protein, inhibition of myosin II ATP hydrolysis, disruption of actin polymerization, and actin stabilization."
"ROCKII inhibition, myosin II inhibition, actin polymerization inhibition and actin stabilization [stopped] flow-induced SOX9 expression"
"LPA treatment[LPA activates RhoA] did not alter Sox9 basal expression levels and treated cells maintained their ability to upregulate SOX9 1.4±0.09-fold with oscillatory fluid flow, indicating that RhoA may not be a direct inhibitor of chondrogenic differentiation"
"inhibiting tension within the actin cytoskeleton promotes chondrogenic differentiation; however, an intact cytoskeleton is necessary for flow-induced alterations in Sox9 expression."
Gene expression of single human mesenchymal stem cell in response to fluid shear.
"An optical tweezer model has been employed to exert different levels of shear stress on a single non-adherent human bone marrow-derived mesenchymal stem cell to simulate physiological flow conditions. A single-cell quantitative polymerase chain reaction analysis showed that collagen type 1, alpha 2 (COL1A2), heat shock 70-kDa protein 1A (HSPA1A) and osteopontin (OPN){up in LSJL} are expressed to a detectable level in most of the cells. After exposure to varying levels of shear stress, there were significant variations in gene transcription levels across human mesenchymal stem cells derived from four individual donors. Significant trend towards upregulation of COL1A2 and OPN gene expression following shear was observed in some donors with corresponding variations in HSPA1A gene expression. Shear stress associated with vascular flow may have the potential to significantly direct non-adherent stem cell expression towards osteogenic phenotypic expression. these results are influenced by the selection process and donor variability."
"gravity’s impact on fluid flow is the creation of flows due to density differences (buoyancy-induced convection) as well as due to thermal convection. Gravitationally induced bulk convection is a type of natural convection caused by buoyancy variations that result from material properties other than temperature. With gravity, thermal convection occurs when heated fluids rise to the top along the gravity vector, which are then replaced by cooler fluids. Both bulk and thermal convection establish a fluid current in the body that is considered essential to driving mass transport and rapidly dissipating heat"
Gene expression of single human mesenchymal stem cell in response to fluid shear.
"An optical tweezer model has been employed to exert different levels of shear stress on a single non-adherent human bone marrow-derived mesenchymal stem cell to simulate physiological flow conditions. A single-cell quantitative polymerase chain reaction analysis showed that collagen type 1, alpha 2 (COL1A2), heat shock 70-kDa protein 1A (HSPA1A) and osteopontin (OPN){up in LSJL} are expressed to a detectable level in most of the cells. After exposure to varying levels of shear stress, there were significant variations in gene transcription levels across human mesenchymal stem cells derived from four individual donors. Significant trend towards upregulation of COL1A2 and OPN gene expression following shear was observed in some donors with corresponding variations in HSPA1A gene expression. Shear stress associated with vascular flow may have the potential to significantly direct non-adherent stem cell expression towards osteogenic phenotypic expression. these results are influenced by the selection process and donor variability."
Levels of baseline gene expression in cultured hMSC (CD_SEL)
18s COL1A2 COL2A1 ALP ACAN OPN HSPA CBFA1 SOX9 COX2
100% 92% 0% 4% 28% 46% 94% 19% 17% 25%
"The fluid velocity at 20, 40, 60 and 80 µm s−1 corresponds to a shear stress of 0.015, 0.030, 0.045 and 0.060 Pa, respectively."
So isolated shear stress on one stem cell is likely to induce osteogenic differentiation. You need to achieve mesenchymal condensation to likely to induce chondrogenic gene expression.
Structure-function relationships in the stem cell's mechanical world B: emergent anisotropy of the cytoskeleton correlates to volume and shape changing stress exposure.
"In the body, electrostatic forces and energies (e.g., ion pairs, hydrogen bonds) are essential for the interaction of virtually all biological macromolecules. Due to the polar nature of water, the intercellular and intracellular interactions between water and hydrophilic and hydrophobic molecules, including polysaccharides, lipids, and proteins, are critical for healthy physiological processes."
Structure-function relationships in the stem cell's mechanical world B: emergent anisotropy of the cytoskeleton correlates to volume and shape changing stress exposure.
"The spatiotemporal organization of tubulin and actin elements of the cytoskeleton changes in response to volume and shape changing stresses [emulate] those during development, prior to the first beating of the heart or twitching of muscle. [We] quantify the change over baseline in spatiotemporal distribution of actin and tubulin in live C3H/10T1/2 model stem cells subjected to volume changing stresses induced by seeding at density as well as low magnitude, short duration, shape changing (shear) stresses induced by fluid flow (0.5 or 1.0 dyne/cm2 for 30/60/90 minutes). Upon exposure to fluid flow, both tubulin thickness (height) and concentration (fluorescence intensity) change significantly over baseline, as a function of proximity to neighboring cells (density) and the substrate (apical-basal height). Amplification of stress gradients (flow velocity) [occurs] with increasing distance to nearest neighbors and the substrate, i.e. with decreasing density and toward the apical side of the cell, tubulin adaptation appears to depend significantly on the magnitude of the stress to which the cell is exposed locally. Adaptation of actin to the changing mechanical milieu is more global, exhibiting less significant differences attributable to nearest neighbors or boundaries than differences attributable to magnitude of the stress to which the cell is exposed globally (0.5 versus 1.0 dyne/cm2). changes in the actin cytoskeletal distribution correlate positively with one pre-mesenchymal condensation marker (Msx2) and negatively with early markers of chondrogenesis (ColIIaI alone, indicative of pre-hypertrophic chondrogenesis) and osteogenesis (Runx2). Changes in the tubulin cytoskeletal distribution correlate positively with a marker of pericondensation (Sox9 alone), negatively with chondrogenesis (ColIIaI) and positively with adipogenesis (Ppar-gamma 2). Exposure of MSCs to volume and shape changing stresses results in emergent anisotropy of cytoskeletal architecture (structure), which relate to emergent cell fate (function)."
"prescribed seeding conditions as well as seeding density can be used to subject multipotent stem cells (MSCs) to volume changing stresses and that changes in volume of the cell are associated with changes in shape, but not volume, of the cell nucleus."
"Tubulin resists compression and contributes to cell viscosity. In contrast, actin resists tension, contributing to cell stiffness and resistance to deformation"
"in contrast to the tubulin cytoskeleton which acts as a damper, resisting forces that compress the cell, the actin cytoskeleton acts like an elastic rope or spring to resist forces pulling on the cell"
Genes detected and what stage they are markers for: "pre- (Runx2 with Msx2) , peri-mesenchymal condensation (ColIa1, Sox9), chondrogenesis (Sox9 and ColIIa1, and later AGC Aggrecan), osteogenesis (Runx2 without Msx2), adipogenesis (Ppar-γ2)"
"Cells seeded at LD (5,000 cells/cm2) [had] larger appearing cells and more defined actin microfilaments and microtubules than cells seeded at HD (35,000 cells/cm2) "
"The increase in apical[means at the apex or tip] and non-flow side tubulin in a cell is negatively correlated with ColIIaI, a marker of chondrogenesis. The non-flow side of tubulin is also positively correlated with Sox9, a marker of peri-mesenchymal condensation, and an earlier marker of chondrogenesis. The total amount of tubulin shows the same correlation with Sox9"<-So what you would want to do is increase the amount of the flow side tubulin to get both Col2a1 and Sox9.
"exposure to flow completely abrogates[abolishes] aggrecan expression"<-this is strange considering aggrecan was upregulated in LSJL.
"The increase in actin in the flow, non-flow, apical, and basal sides of the cell, is negatively correlated with ColIIaI, a marker of chondrogenesis. It is also negatively correlated with Runx2, a marker of pre-mesenchymal condensation as well as early chondrogenesis and osteogenesis. Furthermore, it is positively correlated with Msx2, a marker of pre-mesenchymal condensation"
Advances in assessment of bone porosity, permeability and interstitial fluid flow
"Gravity strongly affects fluid behavior by creating forces that drive and alter its motion. In the presence of gravity, fluid flow can also lead to altered phase interactions and processes that regulate gases. Controlling fluid flow in the absence of gravity creates both significant and novel challenges, where flow can be significantly complicated by temperature, capillary networks of different geometries, changes in fluid surface tension, droplets, and undesirable bubble formation. The near elimination of buoyancy, hydrostatic pressure and sedimentation cause adjustments to flow dynamics: liquids climb container walls, there is limited drainage of liquids, and liquids of different densities can stratify."
Advances in assessment of bone porosity, permeability and interstitial fluid flow
"Bone tissue contains two types of fluid, blood and interstitial fluid. Through the arterial system blood arrives containing oxygen and nutrients; after passing through the bone capillaries the blood components then depart containing less oxygen and nutrients, and more carbon dioxide and other cellular waste products. Many substances, including amino acids, sugars, fatty acids, coenzymes, hormones, neurotransmitters, and inorganic compounds, are exchanged from the capillaries into the interstitial fluid in the vascular porosity in cortical bone. The vascular porosity (VP) is the space inside the Haversian and Volkmann canals that contains the soft tissue structures, blood vessels and nerves. The mineralized tissue matrix in bone contains lacunar pores and slender canalicular channels, forming a complex network that is called the lacunar–canalicular porosity (LCP). Lacunar pores are occupied by osteocytes, the most abundant cell type in bone, and the canaliculi contain the cell processes emanating from contiguous osteocytes, thus permitting communication between neighboring bone cells."
"Interstitial fluid flow is enhanced by the time-varying mechanical loads applied to bone, causing deformations that result in convection of interstitial fluid in and out of the LCP. The interstitial fluid in the LCP travels inside the canaliculi and lacunae, passing through the space between the osteocyte dendritic process and the canalicular wall, dragging the osteocyte's cell process and deflecting the tethering elements that attach the cell process to the canalicular wall. Focal adhesion complexes and integrins located at the cell process membrane are stretched out, which may lead to the genesis of molecules involved in signaling pathways (e.g., OPG, RANKL, NO, PGE2, ATP, sclerostin, DMP1, FGF23)"
"The largest pore size is associated with the vascular porosity (VP), which consists of the volume of all the tunnels in bone that contain blood vessels and includes all the osteonal canals (primary and secondary) as well as transverse (Volkmann) canals. The lacunar–canalicular porosity (LCP) contains the second largest pore size, and it is associated with the osteocyte lacunae and canaliculi channels. The space between the osteocyte and the lacunar–canalicular wall is filled by the osteocyte's glycocalyx and interstitial fluid. The smallest pore size in bone is found in the collagen-apatite porosity (CAP). The pores are spaced between the collagen and the crystallites of the mineral apatite. At this level, most of the water is bound to ionic crystals in bone. The typical lineal dimension associated with the vascular, lacunar–canalicular, and collagen-apatite porosities is 50 μm, 100 nm, and 1 nm, respectively" The epiphysis contains blood vessels and possibly collagen-apatite so those are the areas of interestitial fluid flow we should be most concerned with for height growth.
Vascular porosity is age dependent and increases with age. Vascular porosity is smaller in mice than humans.
"The pore size of the vascular porosity is sufficiently large to permit a rapid decay of a pressure pulse by diffusion, and thus should be a low-fluid-pressure domain. The fluid pressure magnitude in the vascular porosity domain is considered similar to the blood pressure in bone capillaries (40–60 mmHg, or correspondingly 5.3 kPa–8 kPa) because an interstitial fluid pressure in the vascular porosity significantly greater than 40–60 mmHg would collapse these blood vessels, and a prolonged increase in the interstitial fluid pressure significantly above the blood pressure would deprive the tissue of oxygen{maybe this deprivation of oxygen creates a pro-chondrogenic microenvironment?} and nutrients. The lacunar–canalicular system is a high-fluid-pressure domain because the pore size of the LCP is very small, leading to a slow decay of a pressure pulse. "
"Pressure magnitude gradients produced in bone with permeabilities on the order of 10−20 m2 are required to produce the fluid pressure necessary to convect fluid flow in the lacunar-canalicular system and allow fluid to flow against the trans-cortical pressure gradient. "
Influence of interstitial bone microcracks on strain-induced fluid flow.
"up to one half of bone mass could be lost during a 3-year trip to Mars, resulting in mission-compromising low-energy bone fractures, complications from renal stones caused by skeleton-released calcium and an increased incidence of fragility fractures when returning to full or partial gravity"
Influence of interstitial bone microcracks on strain-induced fluid flow.
"the presence of a microcrack in the interstitial osteonal tissue may drastically reduce the fluid velocity inside the neighbouring osteons. This fluid inactive zone inside osteons can cover up to 10% of their surface. Consequently, the fluid environment of bone mechano-sensitive cells is locally modified."<-Maybe microcracks are part of the conditioning response to LSJL.
"microdamage occurring inside the osteonal volume may generate a cell-transducing mechanism based on ruptured osteocyte processes"<-this mechanism may affect MSCs to differentiate into chondrocytes.
"the fluid velocity induced by cyclic loading is sufficient to stimulate cells [at] ||v|| > 5 × 10−8 m.s−1 where ||v|| designates the fluid velocity vector norm"
"osteocyte apoptosis may play a role in the signalling mechanisms by which bone is remodelled after microcrack formation"
"when a microcrack develops inside the interstitial matrix, the fluid velocities in the closest osteons fall below a threshold value, limiting the osteocyte solicitation and thus initiating the lining cells activation."
Migration of human mesenchymal stem cells under low shear stress mediated by mitogen-activated protein kinase signaling.
" Fluids provide the most fundamental way to transport chemical and biochemical elements within our bodies and apply an essential mechano-stimulus to cells. Furthermore, the cell cytoplasm is not a simple liquid, and fluid transport phenomena together with viscoelastic deformation of the cytoskeleton play key roles in cell function. In microgravity, flow behavior changes drastically, and the impact on cells within the porous system of bone and [are influenced by] an expanding level of adiposity"
Migration of human mesenchymal stem cells under low shear stress mediated by mitogen-activated protein kinase signaling.
"hMSCs are able to detect and respond to shear stress due to blood and interstitial fluid flow through mechanotransduction pathways after transplantation{the idea with LSJL is to get the MSCs to respond by differentiating into chondrocytes}. We examined the effect of shear stress on hMSC migration and the role of mitogen-activated protein kinases (MAPKs) in their migration. Shear stress between 0.2 and 10 Pa, which was produced by the flow medium, was exerted on fluorescently labeled hMSCs. Cell migration was evaluated. hMSCs subjected to a shear stress of 0.2 Pa caused notably faster wound closure than statically cultured hMSCs, while migration in the 0.5- and 1-Pa shear stress group did not differ significantly from that in the control group. Shear stress >2 Pa markedly inhibited hMSC migration. hMSCs subjected to a shear stress of 0.2 Pa displayed an increase in extracellular signal-regulated kinases 1/2 (ERK1/2), c-Jun N-terminal kinases (JNK), and p38 MAPK activation for up to 60 min, while a shear stress of 2 Pa abrogated the activation. JNK and p38 MAPK inhibitors completely abolished the effect of shear stress on hMSC migration, while significant differences were observed between the ERK1/2 inhibitor-treated static control and shear stress groups. Low shear stress effectively induces hMSC migration and that JNK and p38 MAPK play more prominent roles in shear stress-induced migration than ERK1/2."
It's possible also that an inhibition of migration is good as MSCs need to condense to differentiate into chondrocytes.
"laminar shear stress of 0.3 Pa significantly enhanced the migration of human umbilical vein endothelial cells (ECs) compared with a shear stress of 1.2 or 2 Pa"
"Treatment with the JNK inhibitor completely abolished the migratory ability of hMSCs, and shear stress did not compensate for the effect of inhibition. The same phenomenon was observed when p38 MAPK activation was inhibited"
Effect of fatigue loading and associated matrix microdamage on bone blood flow and interstitial fluid flow.
Changes in interstitial fluid flow, mass transport and the bone cell response in microgravity and normogravity
Effect of fatigue loading and associated matrix microdamage on bone blood flow and interstitial fluid flow.
"We investigated the effect of a single period of cyclic fatigue on bone blood flow and interstitial fluid flow. The ulnae of 69 rats were subjected to cyclic fatigue unilaterally using an initial peak strain of -6000 muepsilon until 40% loss of stiffness developed. Groups of rats were euthanized immediately after loading, at 5 days, and at 14 days. The contralateral ulna served as a treatment control, and a baseline control group that was not loaded was also included. After euthanasia, localization of intravascular gold microspheres within the ulna and tissue distribution of procion red tracer were quantified. Microcracking, modeling, and remodeling (Cr.S.Dn, microm/mm(2), Ne.Wo.B.T.Ar, mm(2), and Rs.N/T.Ar, #/mm(2) respectively) were also quantified histologically. Cyclic fatigue loading induced hyperemia[increased blood flow] of the loaded ulna, which peaked at 5 days after loading. There was an associated overall decrease in procion tracer uptake in both the loaded and contralateral control ulnae. Tracer uptake was also decreased in the periosteal region, when compared with the endosteal region of the cortex. Pooling of tracer was seen in microdamaged bone typically adjacent to an intracortical stress fracture at all time points after fatigue loading; in adjacent bone tracer uptake was decreased. New bone formation was similar at 5 days and at 14 days, whereas formation of resorption spaces was increased at 14 days. A short period of cyclic fatigue induces bone hyperemia and associated decreased lacunocanalicular interstitial fluid flow, which persists over the time period in which osteoclasts are recruited to sites of microdamage for targeted remodeling. Matrix damage and development of stress fracture also interfere with normal centrifugal fluid flow through the cortex. Changes in interstitial fluid flow in the contralateral ulna suggest that functional adaptation to unilateral fatigue loading may include a more generalized neurovascular response."
"The number of cycles required to fatigue the ulna was 3076 ± 2584, and the peak load used to induce bone fatigue was − 23.8 ± 3.0 N"
"cyclic mechanical loading decreased osteocyte lacunar staining in both the fatigue-loaded and contralateral control ulna at all time points after loading, when compared with the baseline control group"
"an inverse or complex relationship may exist between bone blood flow and interstitial fluid flow in the lacunocanalicular network of bone over time. The existence of an inverse relationship between bone blood flow and interstitial fluid flow is also supported by studies showing that elevated intramedullary pressure and elevated interstitial fluid flow in bone are induced by reduction of venous blood outflow by femoral vein ligation"
"functional adaptation to unilateral cyclic fatigue loading includes a neurovascular response in the contralateral limb"<-Contralateral adaptation was also reported in LSJL.
This dabbled remodeling is much more specific than the massive hole present in the LSJL drilling study. Intracortical damage is black arrows and bone remodeling is white arrows. A is five days, B is 14 days.
The influence of load repetition in bone mechanotransduction using poroelastic finite-element models: the impact of permeability.
"there is a minimum time of rest between load cycles that is required to maximize fluid motion, which depends on the order of magnitude of the intrinsic permeability. We show that frequency and rest insertion may be optimized to deliver maximal mechanical stimulus as a function of permeability."<-couldn't get this full study yet.
The influence of load repetition in bone mechanotransduction using poroelastic finite-element models: the impact of permeability.
"there is a minimum time of rest between load cycles that is required to maximize fluid motion, which depends on the order of magnitude of the intrinsic permeability. We show that frequency and rest insertion may be optimized to deliver maximal mechanical stimulus as a function of permeability."<-couldn't get this full study yet.
Here's a review paper on fluid shear stress:
"Bone has the power to regenerate and repair constantly throughout the entire life."
How collagen fibers are formed:
"Interstitial fluid (ISF) is a main component of body mass (up to 20%) and is distributed throughout the ECM. It provides cells with nutrients and waste removal and can also be found in cortical and cancellous bone where it fills the porosities within the tissue. The three levels of porosities in bone are: (1) the vascular porosity within the Volkmann canal and the Haversian canals (20 μm radius), (2) the lacunar-canalicular system (LCS), which are the channel structures within the mineralized bone tissue surrounding osteocytes and their processes (0.1 μm radius), and (3) tiny spaces between crystallites of the mineral hydroxapatite and collagen fibers (0.01 μm radius)"
MOre on osteocytes since osteocytes are the cells directly most strongly impacted by fluid flow it is important to know whether there activities can make you taller: