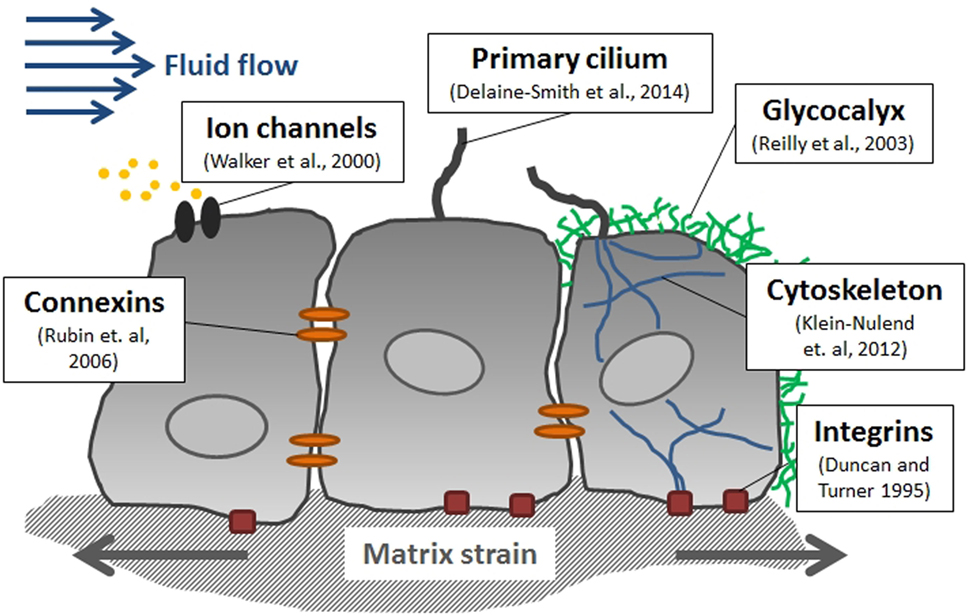
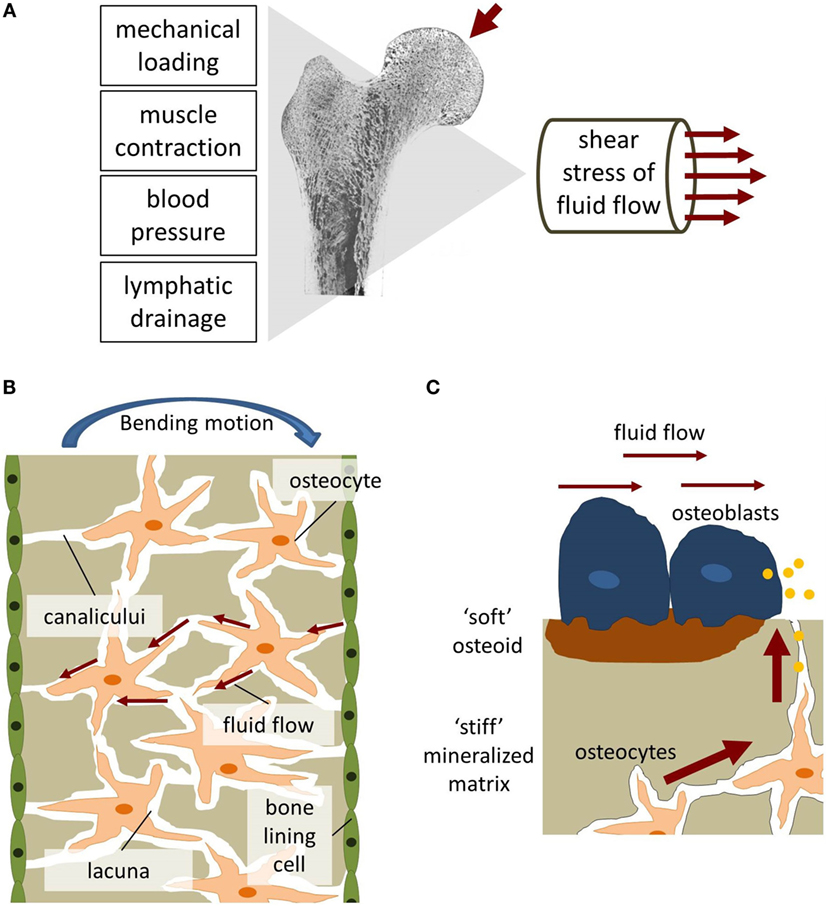
CH Turner was one of the leading researchers behind Lateral Synovial Joint Loading before he passed away and he was the researcher who was most directly interested in bone(Hiroki Yokota for example was more interested in mechanotransduction).
CH Turner's studies are still coming out. Here's one about the gene expression of bone on mechanical loading. It will be interested to compare that to gene expression under LSJL.<-Read that study, it's important!
Gene expression patterns in bone following mechanical loading.
"The primary goal of this study was to determine the time sequence for gene expression in a bone subjected to mechanical loading during key periods of the bone-formation process, including expression of matrix-related genes, the appearance of active osteoblasts, and bone desensitization. A standard model for bone loading was employed in which the right forelimb was loaded axially for 3 minutes per day{so axial loading rather than our lateral loading, note that the epiphysis still gets loading under axial loading as there's no way to avoid it}, whereas the left forearm served as a nonloaded contralateral control. We evaluated loading-induced gene expression over a time course of 4 hours to 32 days after the first loading session. Six distinct time-dependent patterns of gene expression were identified over the time course and were categorized into three primary clusters: genes upregulated early in the time course, genes upregulated during matrix formation, and genes downregulated during matrix formation. Genes then were grouped based on function and/or signaling pathways. Many gene groups known to be important in loading-induced bone formation were identified within the clusters, including AP-1[The C-Fos and c-Jun complex]-related genes in the early-response cluster, matrix-related genes in the upregulated gene clusters, and Wnt/β-catenin signaling pathway inhibitors in the downregulated gene clusters. Chemokine-related genes, were upregulated early but downregulated later in the time course; solute carrier genes{these help with chondrocyte hypertrophy}, were both upregulated and downregulated; and muscle-related genes, were primarily downregulated{This is interesting, as many anabolic pathways are shared between muscle and bone; this gives weight to the theory that muscle and bone compete for resources}."
The Peak Load used was 13N. "Compressive load was applied as an oscillating Haversine waveform for 360 cycles at a frequency of 2 Hz" 24 hours between loading. Genes upregulated in supplementary material were taken from all time points from 4 hours to 32 days. Mice were 20 weeks old.
"Bone responds in an anabolic manner to physiologic dynamic loading. For example, the midshaft humerus in the throwing arm of baseball pitchers and catchers showed enhanced bone mass, structure, estimated strength, and resistance to torsion compared with the nonthrowing control arm.{bone length was measured but the comparative length was not presented, something to ask Stuart J. Warden} In contrast, bone mineral density (BMD) in astronauts decreased 1.0% and 1.5% in the spine and hip, respectively, per month of spaceflight{This is not that much considering astronauts are reported to gain 3 inches in space, this shows you the potential of your intervertebral discs in terms of height gain}"
"Mechanical loading uses pathways currently being investigated for new drug development, such as low density lipoprotein receptor–related protein 5 (LRP5) and sclerostin"<-supplements to look into
"New osteoblasts appear on the bone surface 24 to 48 hours after initiating mechanical loading, and bone formation is observed within 96 hours of loading. Bone formation increases between 5 and 12 days after starting loading, but after 6 weeks of loading, bone formation returns to baseline levels."
This is for osteoblasts not chondrocytes which is what we are mainly looking for with LSJL but it may take 12 days after starting LSJL to notice new osteoblast bone deposition(never mind that LSJL requires a chondrocyte phase beforehand). A month is not enough time to measure results.
The shaft of the bone was used so stem cell genes should be detected. So if any mesenchymal chondrogenesis occurred it should show up.
Looking at what was upregulated in axial Loading and Lateral Loading share many of the same pathways like TGF-Beta and WNT/B-Catenin in addition to many ECM related proteins.
Some genes involved in LSJL that were not involved in axial loading hyaluronan synthase(involved in hyaluronic acid). No MMP3 in axial loading in contrast to lateral loading(MMP-3 is stimulatory to chondrocytes).
And of course no induction of chondrogenic differentiation of stem cells in axial loading(that's more due to hydrostatic pressure though than genes). Though it should be represented by genes which I think it is given the upregulating of ECM genes. Chondrogenic differentiation produces ECM but ECM doesn't always indicate chondrogenic differentiation.
BMP-2 and TGF-Beta were produced by axial loading. Both of which can induce chondrogenic differentiation. Axial Loading + LIPUS may be enough to gain height.
Interleukin 1 receptor-like 1 was expressed by both axial and LSJL. Stat3 was expressed which induces Lin28B expression. It also downregulated FGF23 which may be involved in growth plate reactivation.
Upregulated Genes of Note(see supplementary material):
Acan(upregulated in LSJL)
ADAMTS1(up)
Adh7(up)
Angptl2(up)
Apcs(down)
Apln(up)
Arg1(up)
Bambi
Bgn(up)
BMP2(up)
c1qtnf5(up)
c3ar1(up)
Capn6(up)
Car8(up)
ccl2(up)
ccl7(up)
cd14(up)
cd276(up)
CCND1
Cdh15(down)
Cgref1(up)
Chgb(down)
Cit(down)
Cntn1(up)
Fn1
Follistatin
Cntn1(up in LSJL)
Col2A1 alpha1(up in LSJL)
Col3A1 alpha1(up in LSJL)
Col16A1(up)
crabp2(up)
creb3l1(up)
cspg4(up)
cthrc1(up)
cxcl1(up)
dbx1(down)
dlg4(up)
dnm1(up)
ENPP3(down)
fkbp10(up)
Gfpt2(up)
ggcx(up)
glrb(up)
GPR180(down in LSJL)
grin2d(up)
Hapln1(up in LSJL)
Hdlbp(down)
Hif1alpha
Hnf4a(down in LSJL)
HTRA1(up)
Id2(down in LSJL)
il1rl1(up)
IRS-1(down in LSJL)
Junb(also upregulated in LSJL)
Kcnn2(up)
Lepre1(up)
Leptin
lmna(up)
lox(up)
Lrat(up)
Mall(up)
Metrnl(down)
MMP2(up in LSJL)
MMP9
MMP14(up in LSJL)
NDRG4(up)
Neurod2(down)
Ninj1(down)
Nkx2.5
Nos3
Nr4a2(up)
Pacsin1(down)
Pcdhb2(up)
pcsk6(up)
pdgfc(up)
pdpn(up)
prrx1(up)
prss35(up)
PTHR1
PTGS2(up in LSJL)
PTN(up in LSJL)
RPL36al(down)
S100A4(up)
Scn1a(up)
Sct(down)
Serpina3n(up)
Serpine1(up)
Sept5(down)
Slc1a4(up)
Slc6a2(up)
Slc6a15(up)
Slco2a1(up)
Smad9(up)
Smpd3(down)
SOCS2(Anti-height gene)
SOCS3(upregulated in LSJL)
Sp7
Stat3
Syndecan 4(also upregulated in LSJL)
TGFbp1
TGFbp3
tnfrsf12a(up)
TIMP1(up in LSJL)
Vcan(up in LSJL)
VDR
Zfp36(upregulated in LSJL)
Axial loading upregulated a few chondrogenic genes like Acan and COL2A1 but nowhere near the amount of Collagens upregulated by LSJL which also upregulated Col9. Also key, is that Sox9 is not upregulated in axial loading whereas it is in LSJL.
Downregulated genes of note:
Acacb(down)
Acsl6(down)
Anxa3(down in LSJL)
Arl6ip1(down)
Asb2(down)
Asph(up)
BMPR1B(up in LSJL)
Btla(up)
ccnb1(down in LSJL)
ccr1(up)
dpp4(down)
Egr1(up in LSJL)
Fgr(down)
Fnbp1(down)
GADD45A
Galc(down)
Gas6
Ghitm(down)
GHR
IGFBP6(up in LSJL)
Kynu(up)
Leptin Receptor
Mkrn1(down)
Mrps18b(up)
Myl1(up)
Nexn(down)
Ntn1(up)
Pcsk1(up)
Pdlim3(up)
Pkia(up)
Plag1
Ppp1r3c(up)
Prkaa2(down)
Prkg2(up in LSJL)
Pygl(down)
Rsad2(down as Pcaf)
Sdpr(down)
Sla(down)
Slc16a1(down)
Slc25a30(down)
Sost
Srpkg3(down)
TGFBR3
Tnnt3(down)
Trim55(down)
Tsc22d3(down)
Ucp2(down)
Vav1(down)
Vcam1(down in LSJL)
The differential expression of Egr and BMPR1B between Axial Loading and Lateral Loading could be key to LSJL's ability to induce chondrogenesis.
The gene expression data for LSJL was taken 1 hour after the last loading and in this study genes were taken 4 hours after the first loading. So we can compare these early response genes to see how they compare to LSJL.
Upregulated:
Fosl1
Junb(up in LSJL)
Anxa2
S100A4(up)
S100A10
CCBP2
CCL2(up)
CCL7(up)
CXCL1(up)
CXCL13
IL1RL1(up)
IL1RL2
Osm
Osmr
Socs3(up)
Stat3
Tnfrsf12a(up)
Adamts1(up)
ECM1
Serpina3n(up)
Serpine1(up)
Tfpi2
CCND2
Clic1
Gpr1
KCNE4
Lep
Syndecan4 (up)
Regulatory mechanisms in bone following mechanical loading.
"The right forelimb [of rodents] was loaded axially for three minutes per day, while the left forearm served as a non-loaded, contralateral control. Animals were subjected to loading sessions every day, with 24 hours between sessions. Ulnas were sampled at 11 time points, from 4 hours to 32 days after beginning loading."
Mice were 20 weeks old.
"The peak load achieved during loading was 13 N"
Stat5B was upregulated 4 hours following loading. Stat5b is downregulated in LSJL
The expression of COL1 differs greatly with LSJL. At 14 days there was almost no COL1 expression whereas at 14 days there was still extremely high COL1 levels with Axial Loading.
The expression of COL1 differs greatly with LSJL. At 14 days there was almost no COL1 expression whereas at 14 days there was still extremely high COL1 levels with Axial Loading.
"The CREB-related transcription factors are important for bone formation, specifically ATF4, which is required for collagen synthesis by mature osteoblasts. The CREB motif was predicted to be positive at 2d, 4d, 6d, and 8d. The transcription factors that bind to the CREB motif include cAMP responsive element binding protein 1 (CREB1), cAMP responsive element modulator (CREM), activating transcription factor 1 (ATF1), ATF2, ATF3, ATF4, and ATF7. The CREB motif was present in the promoter of an important matrix gene, fibronectin 1 (Fn1), and in genes that promote collagen construction and cross-linking, including Lox, prolyl 4- hydroxylase beta polypeptide (P4hb), and procollagen C-endopeptidase enhancer (Pcolce)"
"at 32d, the system was less responsive to loading and had shifted from bone forming to baseline bone maintenance"<-maybe every 32 days take a break from LSJL?
Collagen 1 alpha 1 did not begin to rise until two days after loading. The LSJL study took gene expression at 49 days after first loading. In the axial loading Col1A was upregulated 3-fold seven days after loading whereas with LSJL it was upregulated only 2 fold.
Alternative Splicing in Bone Following Mechanical Loading
Alternative splicing means that gene expression was altered in mRNA. The whole bone was ground.
Alternative Splicing in Bone Following Mechanical Loading
Alternative splicing means that gene expression was altered in mRNA. The whole bone was ground.
"Compressive load was applied as an oscillating Haversine waveform for 360 cycles at a frequency of 2 Hz using a Bose ElectroForce 3200 Series electromechanical actuator"<-Peak Load was 13N. Axial loading was used.
"Rats were subjected to loading sessions every day, with 24 hours between sessions." Rats were 20 weeks old. Gene expression data was taken up to 4 hours to 32 days.
The greatest alteration of gene expression occurred at 16 days or about 2 weeks. Maybe this is when conditioning effect starts to inhibit gene expression?
According to this Study Sox9 mRNA was not altered at any time point. Col2a1and Acan mRNA were altered. Tgfbeta1 and Tgfbeta2 expression was altered. The key stature genes HMGA2 and Lin28b were altered in LSJL but not here.
Altered genes of note:
Akt1
Akt2
BMPr1a
BMPr1b{up in LSJL}
BMP2{up in LSJL}
BMP4
CNP
CREB3l1
Esrra
Esrrb
Esr2{up in LSJL}
FGF2{up in LSJL}
FGF4
FGF4
FGF21
FGFR1{up}
FGFR3
GH1{down in LSJL}
GHR
GHRHR
GPC3
HMGA1
ID2{down}
ID4
IGF1
IGF1R
IGF2R
IGF2bp1
NPR1
NPR2
NPR3
PLAG1
PRKG2{up}
RARA
RARA
Runx3
Shh
SHOX2
Smad1{down}
Smad2
Smad3
Smad4
Smad5
Smad7(inhibits BMP signaling, Smad6 which inhibits TGF-Beta signaling is not altered)
Smad9
Sox10
Sox11
Syn3
Twist1
Wnt4
Wnt5a
Here's the Partek GSEA Analysis to compare to LSJL for chondrogenic related genes, Bold means the p-value < 0.05, no fold changes were given and no fold cutoff is used:
Chondroblast Differentiation(6.77) 100%:
RARA
FGF4
FGF2
Cyr61
Chondrocyte Differentiation(5.16) 45%:
Col2a1
Creb3l2
MAPK14
Col11a2
TGFB1
Mef2d
FGFR1
OSR1
FGF9
Cartilage Condensation(8.32) 58.32%:
THRA
COL2A1
Tgfb2
Uncx
Ctgf
Bmpr1b
Acan
Chondrocyte Development(1.80) 33.33%<-this could be a key between LSJL and axial loading which has an enrichment score of 3.80.
Cartilage Development(12.70) 44.2%
Cartilge Development Involved in Endochondral Bone Morphogenesis(2.10) 42.8%
Growth Plate Cartilage Development(0.27) 14.29%
Endochondral Ossification(1.66) 29.41%
Systemic effects of ulna loading in male rats during functional adaptation.
Here's the Partek GSEA Analysis to compare to LSJL for chondrogenic related genes, Bold means the p-value < 0.05, no fold changes were given and no fold cutoff is used:
Chondroblast Differentiation(6.77) 100%:
RARA
FGF4
FGF2
Cyr61
Chondrocyte Differentiation(5.16) 45%:
Col2a1
Creb3l2
MAPK14
Col11a2
TGFB1
Mef2d
FGFR1
OSR1
FGF9
Cartilage Condensation(8.32) 58.32%:
THRA
COL2A1
Tgfb2
Uncx
Ctgf
Bmpr1b
Acan
Chondrocyte Development(1.80) 33.33%<-this could be a key between LSJL and axial loading which has an enrichment score of 3.80.
Cartilage Development(12.70) 44.2%
Cartilge Development Involved in Endochondral Bone Morphogenesis(2.10) 42.8%
Growth Plate Cartilage Development(0.27) 14.29%
Endochondral Ossification(1.66) 29.41%
Systemic effects of ulna loading in male rats during functional adaptation.
"The aim of this study was to determine the effects of loading of a single bone on adaptation of other appendicular long bones and whether these responses were neuronally regulated. Young male Sprague-Dawley rats were used. The right ulna was loaded to induce a modeling response. In other rats, a second regimen was used to induce bone fatigue with a mixed modeling/remodeling response; a proportion of rats from each group received brachial plexus anesthesia to induce temporary neuronal blocking during bone loading. Sham groups were included. Left and right long bones (ulna, humerus, tibia, and femur) from each rat were examined histologically 10 days after loading. In fatigue- and sham-loaded animals, blood plasma concentrations of TNF-α, RANKL, OPG, and TRAP5b were determined. Loading the right ulna induced an increase in bone formation in distant long bones that were not loaded and that this effect was neuronally regulated{LSJL increased length in bones not loaded}. Distant effects were most evident in the rats that received loading without bone fatigue. In the fatigue-loaded animals, neuronal blocking induced a significant decrease in plasma TRAP5b at 10 days. Histologically, bone resorption was increased in both loaded and contralateral ulnas in fatigue-loaded rats and was not significantly blocked by brachial plexus anesthesia. In young, growing male rats we conclude that ulna loading induced increased bone formation in multiple bones. "
"The periosteum is the skeletal tissue with the greatest density of sensory nerve fibers, which are arranged in a dense netlike meshwork that is optimized for detection of mechanical distortion. Nerve branches or single neurons enter the bone cortex, often in association with the microvasculature, and connect individual bone cells to the central nervous system via unmyelinated sensory neurons."
"In the load and block + load groups, loading was performed for 1500 cycles at 4 Hz, with an initial peak strain of −3,750 µɛ (−18 N entered into materials testing machine, −16.8 N applied to ulna). In the fatigue and block + fatigue groups, cyclic loading was performed at 4 Hz. Loading was initiated at −16 N, and the load applied to the ulna was increased incrementally until fatigue was initiated. Loading then was terminated when 40% loss of stiffness was attained."
"TRAP5b is expressed on both immature and mature osteoclasts; plasma TRAP5b concentrations are proportional to osteoclast number."
(*NEW*)
Healing of non-displaced fractures produced by fatigue loading of the mouse ulna.
"Using adult (5 month) C57Bl/6 mice, we first determined that cyclic compression of the forelimb under load-control leads to increasing applied displacement and, eventually, complete fracture. We then subjected the right forelimbs of 80 mice to cyclic loading (2 Hz; peak force approximately 4N) and limited the displacement increase to 0.75 mm (60% of the average displacement increase at complete fracture). This fatigue protocol created a partial, non-displaced fracture through the medial cortex near the ulnar mid-shaft, and reduced ulnar strength and stiffness by >50%. Within 1 day, there was significant upregulation of genes related to hypoxia (Hif1a) and osteogenesis (Bmp2, Bsp) in loaded ulnae compared to non-loaded, contralateral controls. The gene expression response peaked in magnitude near day 7 (e.g., Osx upregulated 8-fold), and included upregulation of FGF-family genes (e.g., Fgfr3 up 6-fold). Histologically, a localized periosteal response was seen at the site of the fracture; by day 7 there was abundant periosteal woven bone surrounding a region of cartilage. From days 7 to 14, the woven bone became denser but did not increase in area. By day 14, the woven-bone response resulted in complete recovery of ulnar strength and stiffness, restoring mechanical properties to normal levels. In the future, the fatigue loading approach can be used create non-displaced bone fractures in transgenic and knockout mice to study the mechanisms by which the skeleton rapidly repairs damage."
Monotonic loading: "Both forelimbs of five mice were loaded by a displacement ramp (0.5 mm/sec) to complete, displaced fracture in order to determine monotonic mechanical properties. Mice were euthanized immediately after loading. Ultimate force (mean ± SD) was 4.32 ± 0.21 N, and stiffness was 3.98 ± 0.26 N/mm."
Fatigue loading: "Both forelimbs of 14 mice were cyclically loaded at peak compressive forces (F) ranging from 2.1 to 3.5 N (50 to 80% of average ultimate force) until complete fracture. "
Force loading, to partial non-displaced fracture: "Right forelimbs of 80 mice were cyclically loaded at peak forces ranging from 3.75 to 4.10 N (70–75% of ultimate force) while displacement was monitored. Loading was terminated when peak displacement increased by 0.75 mm relative to the peak displacement at cycle 10."
The key here is to see if any chondrogenic genes were upregulated in partial, non -displaced fractures(so like a microcrack). Col2a1 was highly upregulated at day 7. BMP2[1.1-1.9 fold] and FGF2[1.1-1.8 fold] were moderately upregulated. FGF2 was more highly upregulated in LSJL than here whereas BMP2 was more highly upregulated here than LSJL. Hif1a the chondrogenically related transcription factor was more significantly upregulated peaking at 3.0 at day 3 and increasing before and decreasing after.
It should be noted that LSJL gene expression was done by microarray whereas this study was done with RT-PCR with the exception of BMP2 which was also done by PCR.
Tibial loading increases osteogenic gene expression and cortical bone volume in mature and middle-aged mice.
(*NEW*)
Healing of non-displaced fractures produced by fatigue loading of the mouse ulna.
"Using adult (5 month) C57Bl/6 mice, we first determined that cyclic compression of the forelimb under load-control leads to increasing applied displacement and, eventually, complete fracture. We then subjected the right forelimbs of 80 mice to cyclic loading (2 Hz; peak force approximately 4N) and limited the displacement increase to 0.75 mm (60% of the average displacement increase at complete fracture). This fatigue protocol created a partial, non-displaced fracture through the medial cortex near the ulnar mid-shaft, and reduced ulnar strength and stiffness by >50%. Within 1 day, there was significant upregulation of genes related to hypoxia (Hif1a) and osteogenesis (Bmp2, Bsp) in loaded ulnae compared to non-loaded, contralateral controls. The gene expression response peaked in magnitude near day 7 (e.g., Osx upregulated 8-fold), and included upregulation of FGF-family genes (e.g., Fgfr3 up 6-fold). Histologically, a localized periosteal response was seen at the site of the fracture; by day 7 there was abundant periosteal woven bone surrounding a region of cartilage. From days 7 to 14, the woven bone became denser but did not increase in area. By day 14, the woven-bone response resulted in complete recovery of ulnar strength and stiffness, restoring mechanical properties to normal levels. In the future, the fatigue loading approach can be used create non-displaced bone fractures in transgenic and knockout mice to study the mechanisms by which the skeleton rapidly repairs damage."
Monotonic loading: "Both forelimbs of five mice were loaded by a displacement ramp (0.5 mm/sec) to complete, displaced fracture in order to determine monotonic mechanical properties. Mice were euthanized immediately after loading. Ultimate force (mean ± SD) was 4.32 ± 0.21 N, and stiffness was 3.98 ± 0.26 N/mm."
Fatigue loading: "Both forelimbs of 14 mice were cyclically loaded at peak compressive forces (F) ranging from 2.1 to 3.5 N (50 to 80% of average ultimate force) until complete fracture. "
Force loading, to partial non-displaced fracture: "Right forelimbs of 80 mice were cyclically loaded at peak forces ranging from 3.75 to 4.10 N (70–75% of ultimate force) while displacement was monitored. Loading was terminated when peak displacement increased by 0.75 mm relative to the peak displacement at cycle 10."
The key here is to see if any chondrogenic genes were upregulated in partial, non -displaced fractures(so like a microcrack). Col2a1 was highly upregulated at day 7. BMP2[1.1-1.9 fold] and FGF2[1.1-1.8 fold] were moderately upregulated. FGF2 was more highly upregulated in LSJL than here whereas BMP2 was more highly upregulated here than LSJL. Hif1a the chondrogenically related transcription factor was more significantly upregulated peaking at 3.0 at day 3 and increasing before and decreasing after.
It should be noted that LSJL gene expression was done by microarray whereas this study was done with RT-PCR with the exception of BMP2 which was also done by PCR.
Here's what a bone microfracture-microcrack looks like:
M stands for marrow. CB stands for cortical bone. WB stands for woven bone.
"Longitudinal sections of fatigue-loaded ulnae (H&E) show that the fracture occurred as a non-displaced, oblique crack through the medial cortex (arrows). On day 1 after loading, a clot is seen on both ends of the crack. On day 3, the periosteum is expanded and filled with cellular, fibrovascular tissue; nascent woven bone is seen sub-periosteally. On days 7 and 11 there is abundant woven bone on the medial periosteum. In approximately one-half of specimens the callus contained no cartilage (not shown), but in the others there was cartilage (*) in the center of the woven bone."
"Cyclic loading of the rat forelimb (~18 N peak force) to 85% of fracture displacement resulted in a non-displaced fracture localized to the medial cortex of the ulna and an associated loss of ulnar strength and stiffness of 55 and 80%, respectively. Because the fracture in the rat ulna was partial and non-displaced, and because the repair process involved negligible cartilage formation, we referred to this as a “stress fracture”, consistent with descriptions by others"<-now we've considered that that 0.5N in LSJL is equivalent to 100N on a 200lbs human which is already a challenge. Imagine the challenge of generating 18N peak force. It's possible that less force may be needed if the force is applied cyclically over a long period of time as long as that force causes residual damage in the bone.
"Cartilage was often observed at 7 and 11 day timepoints and appeared only on the medial surface, corresponding to the periosteal fracture location. By comparison, in studies of complete fracture in mice cartilage is seen on both sides of the bone as well as between the fractured ends"
The fracture occurred on the medial side and although the majority of the activity is on the medial side there is some enhanced activity on the lateral side giving weight to the possibility of gradually lengthening the bone through microfracture(as the unfractured side does seem to adapt). Although the force required to induce a sufficient microfracture may be too large to be induced under normal physiological circumstances(and you would notice a bone adaptation as large as that depicted). That does not preclude the possibility that rapid loading that is large enough to induce residual damage to bone is enough to induce such a microfracture as well. Something like tapping.
Tibial loading increases osteogenic gene expression and cortical bone volume in mature and middle-aged mice.
"We examined this question in female BALB/c mice of different ages, ranging from young to middle-aged (2, 4, 7, 12 months). We first assessed markers of bone turnover in control (non-loaded) mice. Serum osteocalcin and CTX declined significantly from 2 to 4 months. There were similar age-related declines in tibial mRNA expression of osteoblast- and osteoclast-related genes, most notably in late osteoblast/matrix genes. For example, Col1a1 expression declined 90% from 2 to 7 months. We then assessed tibial responses to mechanical loading using age-specific forces to produce similar peak strains (-1300 µε endocortical; -2350 µε periosteal). Axial tibial compression was applied to the right leg for 60 cycles/day on alternate days for 1 or 6 weeks. qPCR after 1 week revealed no effect of loading in young (2-month) mice, but significant increases in osteoblast/matrix genes in older mice. For example, in 12-month old mice Col1a1 was increased 6-fold in loaded tibias vs. controls. In vivo microCT after 6 weeks revealed that loaded tibias in each age group had greater cortical bone volume (BV) than contralateral control tibias, due to relative periosteal expansion. The loading-induced increase in cortical BV was greatest in 4-month old mice (+13%). Non-loaded female BALB/c mice exhibit an age-related decline in measures related to bone formation. Yet when subjected to tibial compression, mice from 2-12 months have an increase in cortical bone volume. Older mice respond with an upregulation of osteoblast/matrix genes, which increase to levels comparable to young mice."
Unfortunately, no chondrogenic genes were studied.
Global gene expression analysis in the bones reveals involvement of several novel genes and pathways in mediating an anabolic response of mechanical loading in mice
"We applied mechanical loads[4-point bending] to the right tibias of the B6 mice at 9 N, 2 Hz for 36 cycles per day, with the left tibias used as unloaded controls"
"4 days of loading"
"Twenty-four hours after last stimulation"<-whereas LSJL was 1 hour after last stimulation.
"Ten-week-old C57BL/6J female mice"
Complete list of supplementary gene comparison to LSJL to be done. Gene comparison of just genes on main paper and spot comparisons done below.
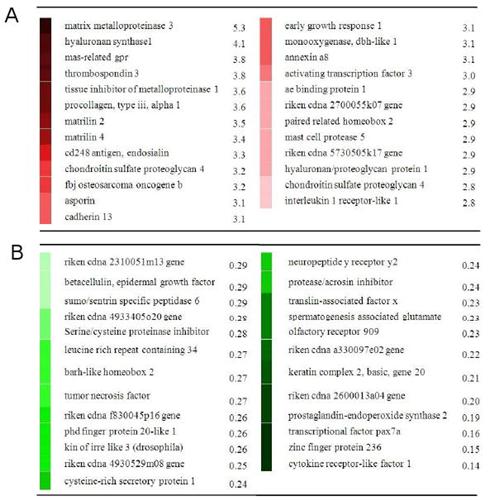
Genes upregulated in bone to four point bending also upregulated by LSJL:
Ptn
Ogn{down}
Itm2a
Lepre1
Col6a3
Col14a1
Col18a1
Matn2
Lox
Gas1
Timp1
Acta2
Ppfibp1{down}
Fer1l3
Spon2
Wnt2
Lmna
Sgk
Odz3
Anxa8
Chl1
Adamts4
Tcf12{down}
Col4a2
Junb
Tnc{down}
Bgn
Egfr
MMP2
BSP
Downregulated:
Mkrn1
Global gene expression analysis in the bones reveals involvement of several novel genes and pathways in mediating an anabolic response of mechanical loading in mice
"We applied mechanical loads[4-point bending] to the right tibias of the B6 mice at 9 N, 2 Hz for 36 cycles per day, with the left tibias used as unloaded controls"
"4 days of loading"
"Twenty-four hours after last stimulation"<-whereas LSJL was 1 hour after last stimulation.
"Ten-week-old C57BL/6J female mice"
Complete list of supplementary gene comparison to LSJL to be done. Gene comparison of just genes on main paper and spot comparisons done below.
Genes upregulated in bone to four point bending also upregulated by LSJL:
Ptn
Ogn{down}
Itm2a
Lepre1
Col6a3
Col14a1
Col18a1
Matn2
Lox
Gas1
Timp1
Acta2
Ppfibp1{down}
Fer1l3
Spon2
Wnt2
Lmna
Sgk
Odz3
Anxa8
Chl1
Adamts4
Tcf12{down}
Col4a2
Junb
Tnc{down}
Bgn
Egfr
MMP2
BSP
Downregulated:
Mkrn1